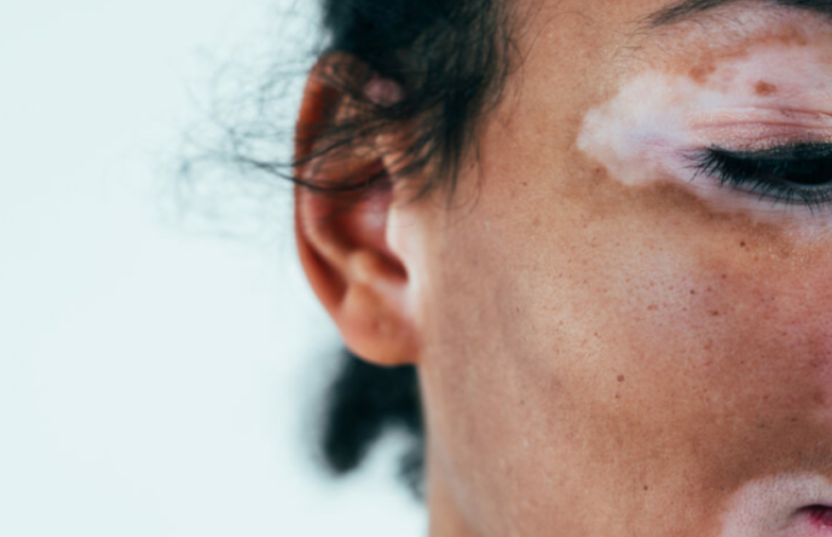
Ruxolitinib Rejected: NICE’s Decision on Vitiligo — and What Comes Next
On 10 July 2025, the UK’s National Institute for Health and Care Excellence (NICE) formally announced its decision not to recommend ruxolitinib cream for people with non-segmental vitiligo on the NHS — a decision that comes after nearly two years of public consultation, committee reviews, and a high-profile appeal.
The outcome has left many in the vitiligo community devastated. For the first time in decades, a licensed treatment had been developed that offered real repigmentation potential. Yet once again, people with vitiligo have been told they must wait.
Despite overwhelming patient and clinical support, NICE has chosen not to recommend ruxolitinib — not because the treatment doesn’t work, but arguably because the system used to evaluate it is deeply flawed. This decision reflects a wider failure to properly value treatments for visible, life-altering conditions like vitiligo. NICE cited uncertainty around comparisons to unlicensed treatments, doubts over long-term effectiveness, and a lack of measurable benefit using the EQ-5D — a tool that is simply not designed to reflect the emotional and social trauma our community lives with every day.
The pain of vitiligo is not just skin deep. It affects identity, confidence, and mental health — yet this is not captured by traditional economic modelling. This outcome shows that the lived reality of patients is still too easily discounted when numbers don’t align neatly with standard frameworks.
We believe this decision fails to recognise both the urgency of unmet need and the opportunity to do better. But it has strengthened our resolve. We will continue working with stakeholders, researchers, and pharmaceutical partners to challenge these systemic gaps — and to make sure future treatments are judged on what they truly mean to the people who need them.
– Abigail Hurrell, Director, The Vitiligo Society
This blog offers a comprehensive look at what happened, why, and what the future holds. It is informed by formal committee documents, appeals, internal communications, and hundreds of patient testimonies submitted as part of our campaign.
Timeline of the Ruxolitinib Appraisal
| Date | Event |
|---|---|
| Nov 2021 | Appraisal referral received by NICE |
| Mar 2023 | Scoping document published for consultation |
| July 2024 | Draft guidance issued: ruxolitinib not recommended |
| Oct 2024 | Formal appeal submitted by patient and clinical stakeholders |
| Dec 2024 | Appeal upheld on three points |
| May 2025 | Final Appraisal Committee Meeting held |
| 10 July 2025 | Final decision published — treatment still not recommended |
| 24 July 2025 | Appeal submission deadline |
What Is Ruxolitinib and Why It Offers Hope
Ruxolitinib cream, marketed as Opzelura by Incyte, is a topical JAK inhibitor licensed to treat non-segmental vitiligo affecting the face and body in patients aged 12 and up. It is the first treatment of its kind approved by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for this condition.
Already licensed in the US and EU, ruxolitinib offers an at-home treatment that has shown significant results in helping patients regain skin pigmentation — particularly on the face.
The results are promising. UK patients deserve the same chance as those in Europe.
– UK Dermatologist

Why This Matters: The Patient Reality
For decades, people living with vitiligo have faced inadequate, inconsistent, and often ineffective treatment options. This is not a cosmetic concern — vitiligo is a chronic autoimmune condition with profound psychological, social, and physical impacts.
During the campaign, we received thousands of submissions from patients. Many were harrowing.
It has absolutely destroyed me. I struggle every single day, to the point I no longer want to be here. I cry nearly every day and wish it would just go away.
– Patient submission to NICE, April 2025
Research commissioned by The Vitiligo Society highlights the scale of this impact:
- 79% said vitiligo negatively impacted their appearance.
- 63% reported mental health deterioration.
- 41% struggle with self-esteem.
- Only 1% of people using NHS treatments considered them “very successful”.
Patients also raised urgent concerns about groups disproportionately affected by the condition — and for whom this decision may be especially devastating:
- People of colour, who experience more visible contrast in pigmentation, increased stigma, and limited access to camouflage options.
- Young people, who face bullying, isolation, and suicidal thoughts during critical stages of identity formation.
- Lower-income families, who are unable to afford private care and now face continued exclusion from treatment options.

A Summary of the Key Decision Points
Despite strong patient support and evidence of clinical benefit, NICE ultimately decided not to recommend ruxolitinib for NHS use. Below, we break down the key reasons behind the decision — based on NICE’s final guidance and appraisal documents.
1. Uncertainty Around Comparative Effectiveness: NICE concluded that while ruxolitinib showed clinical benefit versus placebo, there was too much uncertainty when comparing it to other treatments currently used in the NHS — such as:
- Topical corticosteroids
- Calcineurin inhibitors
- Narrowband UVB phototherapy (though not available to all patients)
NICE felt the data didn’t robustly show that ruxolitinib was significantly better than these unlicensed treatments, particularly for longer-term outcomes.
2. HRQoL Gains Were Not Adequately Captured: NICE uses QALYs (quality-adjusted life years) to assess value — requiring utility values that can be plugged into economic models.
- Ruxolitinib showed improvements in patient-reported outcomes, but not in EQ-5D utility scores, the standard tool NICE prefers.
- NICE found these improvements were not large or consistent enough to confidently translate into QALY gains.
- Alternative measures like VitiQoL were discussed but not accepted for base-case modelling.
This is a major barrier — because without quantifiable QALY gain, the treatment’s benefit is undervalued in cost-effectiveness terms.
3. Cost-Effectiveness Threshold Was Exceeded: Even after Incyte submitted a revised Complex PAS (capping NHS costs at 5 tubes/year), NICE estimated the incremental cost-effectiveness ratio (ICER) remained above their typical threshold. Reasons included:
- High acquisition cost of the drug
- Modest or uncertain QALY gains
- Uncertainty in long-term duration of benefit
Ultimately, NICE concluded that the value for money didn’t meet their threshold under their standard methods.
4. Use of Phototherapy as a Comparator: Despite known issues with access, NICE insisted that phototherapy be included as a comparator, since it is listed in treatment guidelines.This hurt ruxolitinib’s position because:
- NICE assumed some patients could access phototherapy, making ruxolitinib appear more expensive in comparison.
- Access disparities (geographic, socioeconomic, racial) were acknowledged but not enough to dismiss phototherapy as a comparator.
5. Wider Societal and Equity Concerns Not Prioritised: NICE acknowledged:
- Vitiligo can cause severe psychological distress
- Disproportionate impact on people of colour and young people
- Strong patient voice and unmet need
However, NICE stated that these equity concerns did not outweigh the cost-effectiveness concerns, and could not be used alone to justify approval.
For more information read our supplementary blog: Understanding the Decision Behind the Ruxolitinib Final Draft Guidance. A Q&A with the decision makers or visit the NICE website.
Where the Appeal Succeeded — and Failed
In October 2024, The Vitiligo Society and other stakeholders submitted a formal appeal to NICE after the initial rejection. In December 2024, three of those appeal points were upheld, including:
- That the original committee did not adequately consider the impact on people with protected characteristics, especially Black and Asian patients.
- That the patient voice was underrepresented in the committee’s judgment.
NICE then held a third and final Appraisal Committee Meeting (ACM3) in May 2025. Updated submissions were made, including:
- A revised complex Patient Access Scheme (PAS) from Incyte, limiting NHS cost exposure by capping usage at 5 tubes per year.
- New evidence and statements on racial inequality, access barriers to phototherapy, and gaps in standard care.
- Independent economic and clinical reviews.
Despite this, the final recommendation remained unchanged.

NICE’s Position
NICE has today (Thursday, 10 July) published final draft guidance not recommending ruxolitinib for treating non-segmental vitiligo in people 12 years and over. Ruxolitinib is a cream which is a licensed treatment for non-segmental vitiligo that affects the face. Following our initial negative draft guidance being published last year the company and several stakeholders appealed the decision, and an appeal hearing was held in October 2024.
The appeal upheld several points and NICE’s independent committee held a third meeting in May this year to fully consider those points, and any additional evidence or information related to the upheld appeal points. Unfortunately, the cost-effectiveness estimates for the treatment remain uncertain because of limitations in the economic model. The most likely cost-effectiveness estimate is higher than what NICE considers an acceptable use of NHS resources.
There is an option for NICE to look at the drug through its rapid review process, whereby ruxolitinib could be reviewed again. Alongside our colleagues at NHS England and other organisations we are always open to discussions on how we can ensure the NHS is able to offer people new treatments which balance the best care with value for money.
NHS England’s Position
Behind the scenes, NHS England has expressed support for finding a commercial solution. In a communication to The Vitiligo Society a representative from NHS England stated:
In parallel to NICE issuing negative final draft guidance today, NHS England and ruxolitinib cream (Opzelura®) manufacturer, Incyte are continuing to explore alternative commercial options to try to enable NHS access to this treatment for people with non-segmental vitiligo in England. There is an established process for NICE guidance to be subject to a rapid review, which does not require a full re-appraisal and is therefore substantially quicker, that could result in a change to a positive NICE recommendation if a feasible and cost-effective commercial agreement can be reached
These commercial conversations are still ongoing and The Vitiligo Society will remain in contact with NHS England and Incyte to keep updated on progress and continue to press for treatment availability for the vitiligo community.
The Drug Manufacturer’s Position
Speaking to The Vitiligo Society, Incyte, the manufacturer of ruxolitinib, has expressed deep disappointment at NICE’s final decision — especially given that the treatment is already being made available across a growing number of European countries
We are disappointed by NICE’s negative Final Draft Guidance on the reimbursement of ruxolitinib cream for the treatment of non-segmental vitiligo in England and Wales. People living with vitiligo have long hoped for an effective, licensed treatment for a condition that can be highly stigmatizing, as we’ve heard directly from patients throughout the appraisal process.
A growing number of European countries have recognized the value of providing access to the first and only licensed treatment for vitiligo, with some even enabling fast-track access. This decision raises serious concerns that patients in England and Wales may face a lower standard of care than their European counterparts.
We remain committed to working collaboratively toward a solution that ensures equitable access to innovation for people living with vitiligo in England and Wales, consistent with what is available across Europe.
~ Hervé Lamarque, Division Vice President, Head of Business Unit, IAI International
Pete Williams, General Manager UK & Ireland and Head of Mid-Size Markets at Incyte, echoed these concerns — while also thanking the vitiligo community for its sustained involvement in the appraisal process:
We are disappointed by the decision taken by NICE to not recommend ruxolitinib cream for the treatment of non-segmental vitiligo in England and Wales. This decision means that many people living with non-segmental vitiligo will remain unable to access an innovative medicine that there is a high level of unmet need for. Throughout the appraisal process, we have remained committed to working with NICE, NHS England and representatives from the patient community to address the burden of this disease
We would like to thank all members of the patient community and their advocates for their continued engagement and participation in the appraisal process — their contributions have been critical in ensuring accurate representation of people living with non-segmental vitiligo and the impact of the condition on their lives. Incyte is dedicated to continuing close and pragmatic collaboration with all relevant stakeholders to support a solution for patients.
These statements reaffirm that industry partners, too, recognise the seriousness of this decision and share in our mission to secure access to innovative, evidence-backed treatments for vitiligo. As stakeholders across the board — from patients to policymakers to pharma — reflect on what comes next, the urgency for reform is clear.

A Turning Point for Future Treatments
This appraisal doesn’t just affect ruxolitinib — it sets a precedent for every vitiligo treatment coming next. More than a dozen promising therapies are in the pipeline, including:
- Phase 3: Povorcitinib, Upadacitinib, Baricitinib, Afamelanotide, Ritlecitinib
- Phase 2: Crisaborole, Deucravacitinib + UVB, Rapamycin, SYHX 1901, Anifrolumab + UVB
If NICE continues to dismiss health-related quality of life (HRQoL) measures that accurately reflect lived experience, these treatments could face the same fate.
The Vitiligo Society is now proactively engaging with pharmaceutical companies now to ensure these future submissions are informed by this process. We are working to improve quality-of-life measurement tools that better capture the full impact of vitiligo — especially in areas EQ-5D fails to reflect.
This rejection, while painful, offers a vital learning opportunity. It shines a light on what must change and gives us a foundation for doing better next time.
Where We Go From Here
After careful consideration and seeking much advice, we have decided not to submit a second appeal.
We exhausted all viable grounds during the first appeal — and several of our key points were upheld, including failures to consider the full impact on people with protected characteristics and the strength of patient voice. However, it has become clear that the final rejection was not based on a lack of public support or patient testimony, but rather on cost-effectiveness modelling and the commercial terms available at the time.
In short: this decision now sits with other stakeholders.
We are:
- Remaining in active communication with NHS England and Incyte, who have both signalled willingness to explore new access routes — including potential revisions to the commercial proposal.
- Working with pharmaceutical companies developing future treatments to help shape clinical trial design, ensure patient-centred data is prioritised, and improve the chances of success in future appraisals.
- Supporting other patient support groups who are calling for a reform of NICE’s evaluation frameworks, so that they better reflect the real-world impact of conditions like vitiligo and the urgent need for innovation.
This is a shift in strategy — not a step back. This rejection, while painful, offers vital insights that will help inform and improve future submissions. And we will continue doing everything we can to ensure those lessons are not lost.
Closing Words
You Are Not Alone. If this decision has left you feeling hopeless or dismissed, know this: your experience is valid, your voice matters, and this community is fighting for change — with you and for you. Please contact our team for more information or support if you need it.
To everyone who shared your story, spoke your truth, and gave your time: you were heard. And your voice will continue to shape the fight ahead. We will not stop until licensed, effective, and accessible treatments for vitiligo are available through the NHS.
Because every patch of lost pigment represents not just skin — but visibility, dignity, and hope.
Please support us in the next stage of this work by becoming a member of The Vitiligo Society. Our core work is funded but just 600 regular donors, and with your support – giving from just £2 a month- we could do so much more, fight so much harder.
Funding disclosure:
The Vitiligo Society has project work supported by an educational grant from Incyte Biosciences UK Ltd. The Vitiligo Society maintains full control over the all project management and content, ensuring independence and impartiality in its work. When it comes to any external funding we follow the current guidance and rules as set out by the Charity Commission and the Fundraising Regulator. We report any research collaborations and/or financial contributions received from industry in our annual reports and accounts as well as being transparent about our partnerships on our website.
Please support our work! You've enjoyed 1 article this month and we hope you have found it useful. Our work is entirely funded by memberships and donations, so please consider joining our charity today and supporting our work.
Already a member? Log in



